Pictures of the Day
3-27-2025
The Molecular Orbital Theory Moment-Review
Some basics of molecular orbital theory as it relates to pi systems. First electrons behave as waves, so they are described by wave functions (also known as orbitals). Adjacent atomic 2p orbitals add according to wave mechanics, namely constructively (in phase) and destructively (out of phase). The in phase addition creates the lower energy bonding molecular orbital (the "hotdog bun"), while the out of phase addition results in the higher energy antiboding orbital. The two electrons end up in the bonding orbital, resulting in what we refer to as a pi bond. Note how these pi bonding electrons in the pi bonding orbital are held out and away from the nuclei, so they provide some partial negative charge (red color) to the electrostatic potential surface. Since electrophiles can interact with these electrons, alkenes react as weak nucleophiles.

Conjugation occurs when adjacent pi bonds overlap, creatiing a large "pi way" for the electrons to delocalize into. The molecule on the right, butadiene, has adjacent pi bonds that overlap to form the large molecular orbital shown. Such overlap of pi bonds is referred to as "conjugation". This is stabilizing compared to isolated pi bonds because pi electrons prefer to be delocalized over as large an area as possible(Golden Rule #7). Since these delocalized pi electrons are in a more stable situation, they are less reactive with electrophiles. The molecule on the left, 1,3 pentadiene, has an sp3 atom between the pi bonds. The pi bonds cannot overlap in this case, so there is no delocalization, no stabilization, and the pi electrons are as reactive as any old alkene.
Once Again, A Movie Ripping Off Chemistry
Enamines ("Mini me") Do you believe me now?
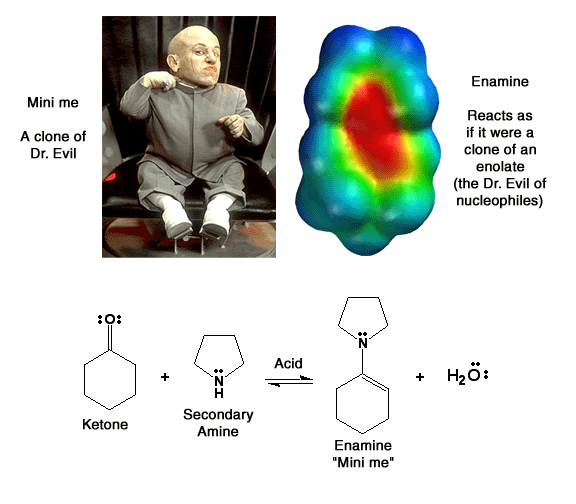
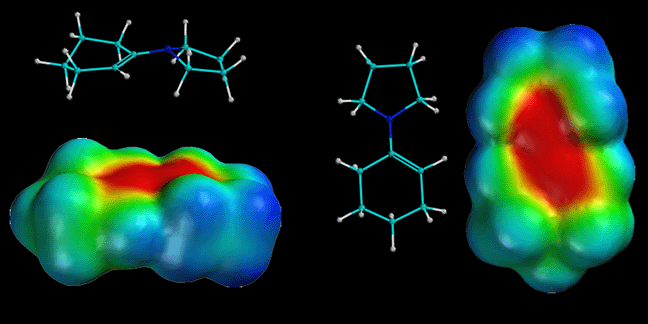
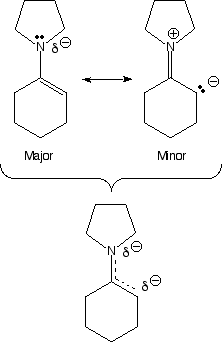
Enamines ("mini me") are molecules derived from ketones and secondary amines, generally pyrrolidine. They react as nucleophiles analogous to enolates ("Dr. Evil"). Note how there is considerable partial negatve charge (red color) on the alpha-carbon atom. This is because of the minor resonance contributor that places negative charge at that position. This partial negative charge makes this carbon nucleophilic, exactly analogous to an enolate. Note that the nitrogen atom is also red, even though the minor contributing structure has a positive charge on the nitrogen. This is not a contradiction because the major resonance contributor has partial negative charge on the nitrogen (due to the lone pair of electrons on nitrogen), and this partial negative charge more than compensates for the small amount of positive charge indicated in the minor contributor. The nitrogen atom does not react as a nucleophile because that would create a less stable product (no motive) compared to reaction at carbon. Complex arguments aside, the important things to remember about enamines are 1) they are formed under mild conditions (it is just a simple Schiff base formation), 2) they react as nucleophiles analgous to enolates woth both alkyl halides and acid chlorides and 3) they are reversibly formed and can be taken off by H3O+ after the reaction. Yea baby!!!





